5) Review Definitions of Competitive Uncompetitive and Mixed Inhibition
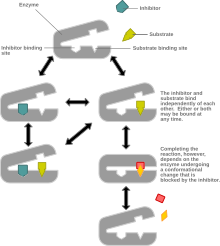

Mixed inhibition is a blazon of enzyme inhibition in which the inhibitor may bind to the enzyme whether or non the enzyme has already bound the substrate but has a greater affinity for one state or the other.[1] It is called "mixed" because it tin can exist seen as a conceptual "mixture" of competitive inhibition, in which the inhibitor can just bind the enzyme if the substrate has not already bound, and uncompetitive inhibition, in which the inhibitor can but bind the enzyme if the substrate has already leap. If the ability of the inhibitor to bind the enzyme is exactly the aforementioned whether or not the enzyme has already leap the substrate, it is known as a non-competitive inhibitor.[1] [2] Non-competitive inhibition is sometimes thought of as a special example of mixed inhibition.
In mixed inhibition, the inhibitor binds to an allosteric site, i.e. a site unlike from the active site where the substrate binds. All the same, non all inhibitors that bind at allosteric sites are mixed inhibitors. [1]
Mixed inhibition may result in either:
In either instance the inhibition decreases the apparent maximum enzyme reaction charge per unit ( ).[iii]
Mathematically, mixed inhibition occurs when the factors α and α' (introduced into the Michaelis-Menten equation to account for competitive and uncompetitive inhibition, respectively) are both greater than 1.
In the special case where α = α', noncompetitive inhibition occurs, in which case is reduced merely is unaffected. This is very unusual in practice.[iii]
Biological examples [edit]
In gluconeogenesis, the enzyme cPEPCK (cystolic phosphoenolpyruvate carboxykinase) is responsible for converting oxaloacetate into phosphoenolpyruvic acid, or PEP, when guanosine triphosphate, GTP, is present. This step is exclusive for gluconeogenesis, which occurs under fasting condition'due south due to the torso'southward depletion of glucose. cPEPCK is known to be regulated by Genistein, an isoflavone that is naturally found in a number of plants. [4] It was showtime proven that genistein inhibits the activity of cPEPCK. In a report, the presence of this isoflavone resulted in a decrease in the level of blood saccharide. A lowered blood saccharide level means less glucose is in the claret. If this occurs in a subject that is fasting, this is because the gluconeogenesis was inhibited, preventing increased production of glucose. The ability of genistein to lower a person'southward blood sugar level allows information technology to exist referred to as an anti-diabetic property. [iv] The mechanism in which genistein inhibited the enzyme cPEPCK was further evaluated. Start, cPEPCK was placed in the presence of 3-Mercaptopropionic acid, or three-MPA, a known inhibitor of the enzyme. It was compared to the results of placing cPEPCK in the presence of genistein, which revealed that the mechanism of mixed inhibition was used to decrease cPEPCK'south activity. [4] cPEPCK undergoes multiple configurations when catalyzing the germination of PEP. Information technology can be either unbound, bound to Gross domestic product or jump to GTP. An experiment that studied the analogousness for genistein in these dissimilar configurations was conducted. Information technology revealed that geinstein favors binding to the cPEPCK with a bound GTP than so the enzyme with a leap GDP, which was found to exist less stable.[4] This was because the GTP-spring cPEPCK revealed an extended binding site for genistein.[iv] This is the same binding site as the enzyme'southward intended substrate, oxaloacetate while the other configurations did not do so in the presence of genistein. [four] This provided evidence that the mechanism of inhibition of cPEPCK by genistein was a mixture of competitive and non-competitive inhibition.
A kallikrein is a type of serine protease, which cleaves peptide bonds after sure amino acids in a protein. These 15 kallikreins, KLK1 to KLK15, are constitute in human tissues. The ability for this molecule to cleave proteins results in the effective activation of prison cell surface receptors, making them crucial elements of many biological indicate transduction pathways, and its amplification through cascades. This family of serine proteases is oftentimes a biomarker to diseases, and therefore, have become a target for inhibition. [5] Inhibition of these kallikreins results in possible therapy for diseases such as metastatic cancer or Alzheimer's disease. [5] Fukugetin, or (+)-morelloflavone, is a type of plant biflavonoid isolated from Garcinia brasiliensis. [5] Afterward isolating fukugetin, it was placed with KLK1, KLK2, KLK3, KLK4, KLK5, KLK6, and KLK7 in varying concentrations.[v] This allowed for the analysis of enzyme kinetics through derivation of parameters Km and Vmax. Through the model of Michaelis-Menten kinetics, the Eadie-Hofstee diagram was plotted.[five] Information technology confirmed that fukugetin acts equally a mixed inhibitor by exhibiting varying but present affinities for the enzyme alone and the enzyme-substrate complex. Analyzing through kinetics, fukugetin decreased the Vmax while it increased the Km for these KLKs.[5] Typically, in competitive inhibition, Vmax remains the same while Km increases, and in non-competitive inhibition, Vmax decreases while Km remains the same. The change in both of these variables is another finding consistent with the effects of a mixed inhibitor.
References [edit]
- ^ a b c "Types of Inhibition". National Institues of Health Chemic Genomics Center. 2011. Archived from the original on 8 September 2011. Retrieved 2 April 2012.
- ^ "Enzyme inhibition". London Southward Depository financial institution University. Archived from the original on 19 March 2012. Retrieved ii April 2012.
- ^ a b Storey KB (2004). Functional Metabolism: Regulation and Adaptation. Wiley-IEEE. p. 12. ISBN978-0-471-41090-4.
- ^ a b c d east f Katiyar SP, Jain A, Dhanjal JK, Sundar D (2015). "Mixed Inhibition of cPEPCK by Genistein, Using an Extended Binding Site Located Adjacent to Its Catalytic Cleft". PLOS ONE. x (xi): e0141987. Bibcode:2015PLoSO..1041987K. doi:10.1371/journal.pone.0141987. PMC4631375. PMID 26528723.
- ^ a b c d e f Santos JA, Kondo MY, Freitas RF, dos Santos MH, Ramalho TC, Assis DM, et al. (March 2016). "The natural flavone fukugetin as a mixed-type inhibitor for human tissue kallikreins". Bioorganic & Medicinal Chemistry Letters. 26 (5): 1485–1489. doi:x.1016/j.bmcl.2016.01.039. PMID 26848109.
Source: https://en.wikipedia.org/wiki/Mixed_inhibition



0 Response to "5) Review Definitions of Competitive Uncompetitive and Mixed Inhibition"
Post a Comment